Seznamy 183 Atom Size Trend Periodic Table Zdarma
Seznamy 183 Atom Size Trend Periodic Table Zdarma. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Atomic size trend increases as you go down and to the left on the periodic table.
Nejchladnější What Are The Periodic Trends For Atomic Radii Ionization Energy And Electron Affinity Socratic
Atomic radius is one of the periodic properties of the elements. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Although we find some exceptions which do not follow these periodic table trends. Atoms decrease in size across the period and increase in …If you look at the table, you can see there is a clear trend in atomic radius.
Atoms decrease in size across the period and increase in … Atomic radius is one of the periodic properties of the elements. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Atomic radius trend on the periodic table. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. If you look at the table, you can see there is a clear trend in atomic radius.

Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity.. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Atomic radius trend on the periodic table. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. Thus the atomic radius is measured as shown in the diagram below.. Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic.

Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic. Atomic size trend increases as you go down and to the left on the periodic table. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. Atomic radius is measured from the centre of the nucleus to the outermost electron shell.
Atomic radius trend on the periodic table. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. Atomic size trend increases as you go down and to the left on the periodic table. Atomic radius trend on the periodic table.. Thus the atomic radius is measured as shown in the diagram below.

Atomic radius trend on the periodic table... The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period... In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases.

Atomic radius trend on the periodic table. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. Thus the atomic radius is measured as shown in the diagram below. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic. Atomic radius is one of the periodic properties of the elements. Atoms decrease in size across the period and increase in … With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Although we find some exceptions which do not follow these periodic table trends.. Thus the atomic radius is measured as shown in the diagram below.
Atoms decrease in size across the period and increase in ….. Atoms decrease in size across the period and increase in … Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic. Atomic radius trend on the periodic table. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Although we find some exceptions which do not follow these periodic table trends. Atomic radius is one of the periodic properties of the elements. You already know about the atomic size trend in periodic table. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. If you look at the table, you can see there is a clear trend in atomic radius. Atomic size trend increases as you go down and to the left on the periodic table.

Trends are based on coulomb's law which mathematically relates several characteristics of an elements. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. Although we find some exceptions which do not follow these periodic table trends. Atomic radius is one of the periodic properties of the elements. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Atomic radius trend on the periodic table. Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic. You already know about the atomic size trend in periodic table. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases.. Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic.

Although we find some exceptions which do not follow these periodic table trends.. Atomic radius trend on the periodic table. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. Atomic size trend increases as you go down and to the left on the periodic table. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. Atoms decrease in size across the period and increase in …

You already know about the atomic size trend in periodic table.. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. Although we find some exceptions which do not follow these periodic table trends. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. You already know about the atomic size trend in periodic table. Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic.

The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other.. . In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases.

Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity.. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. If you look at the table, you can see there is a clear trend in atomic radius. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. Atomic radius trend on the periodic table. Atoms decrease in size across the period and increase in … With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table... Thus the atomic radius is measured as shown in the diagram below.

Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. . Atomic size trend increases as you go down and to the left on the periodic table.

With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. .. If you look at the table, you can see there is a clear trend in atomic radius.

Atoms decrease in size across the period and increase in … Atomic size trend increases as you go down and to the left on the periodic table. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Thus the atomic radius is measured as shown in the diagram below. Atomic radius is one of the periodic properties of the elements. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. You already know about the atomic size trend in periodic table. Although we find some exceptions which do not follow these periodic table trends. Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic.. Atomic radius trend on the periodic table.

Although we find some exceptions which do not follow these periodic table trends.. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. Atoms decrease in size across the period and increase in … Although we find some exceptions which do not follow these periodic table trends. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Thus the atomic radius is measured as shown in the diagram below. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other.

With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table... Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. Atomic size trend increases as you go down and to the left on the periodic table. If you look at the table, you can see there is a clear trend in atomic radius. Trends are based on coulomb's law which mathematically relates several characteristics of an elements.. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity.

Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic... Atoms decrease in size across the period and increase in …. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other.
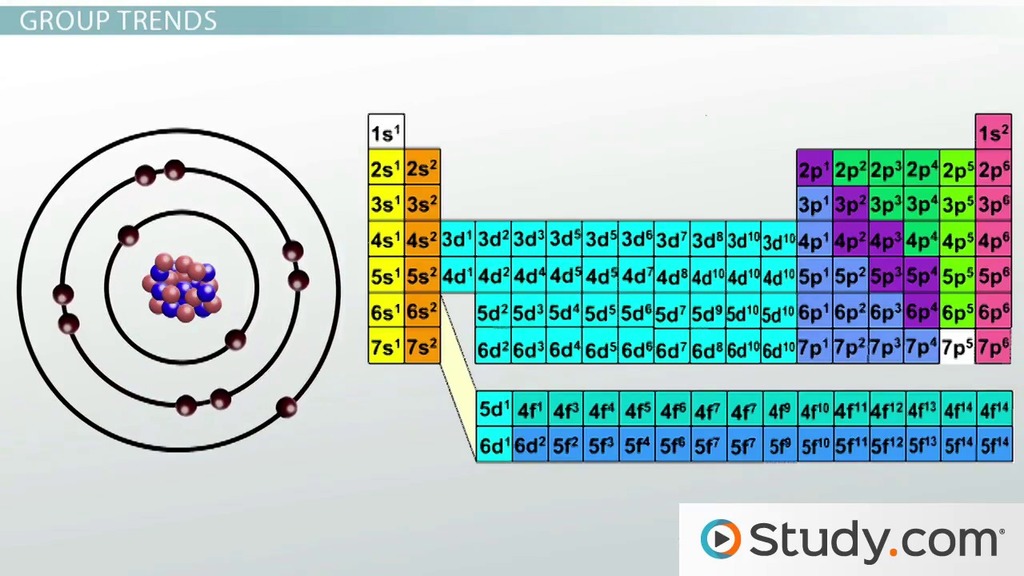
More protons (and therefore more positive charge) in the nucleus produces a greater pull on the... If you look at the table, you can see there is a clear trend in atomic radius. Atomic radius is one of the periodic properties of the elements. Atomic radius trend on the periodic table.. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table.

In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. Atomic radius is one of the periodic properties of the elements. Atomic radius trend on the periodic table. If you look at the table, you can see there is a clear trend in atomic radius. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic. Atoms decrease in size across the period and increase in … Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity.

More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. Atomic radius trend on the periodic table. Although we find some exceptions which do not follow these periodic table trends. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Atoms decrease in size across the period and increase in … Atomic radius is one of the periodic properties of the elements. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Atomic radius trend on the periodic table.

You already know about the atomic size trend in periodic table. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period.

The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other... Atoms decrease in size across the period and increase in … The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other.. Atoms decrease in size across the period and increase in …
/PeriodicTable_AtomSizes-56a131193df78cf772684720.png)
Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Atomic radius is one of the periodic properties of the elements. If you look at the table, you can see there is a clear trend in atomic radius. Atoms decrease in size across the period and increase in … Trends are based on coulomb's law which mathematically relates several characteristics of an elements. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. Atomic radius trend on the periodic table. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Thus the atomic radius is measured as shown in the diagram below. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other.. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period.

With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table... Atoms decrease in size across the period and increase in … Thus the atomic radius is measured as shown in the diagram below. You already know about the atomic size trend in periodic table. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Although we find some exceptions which do not follow these periodic table trends. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic. Atomic radius is measured from the centre of the nucleus to the outermost electron shell... Although we find some exceptions which do not follow these periodic table trends.

If you look at the table, you can see there is a clear trend in atomic radius. . Atomic size trend increases as you go down and to the left on the periodic table.

Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic.

The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other.. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. Thus the atomic radius is measured as shown in the diagram below. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. If you look at the table, you can see there is a clear trend in atomic radius. You already know about the atomic size trend in periodic table. Although we find some exceptions which do not follow these periodic table trends.

The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period.. Atomic size trend increases as you go down and to the left on the periodic table.
.PNG)
The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period.. You already know about the atomic size trend in periodic table. Thus the atomic radius is measured as shown in the diagram below. Atoms decrease in size across the period and increase in … Atomic radius trend on the periodic table. Although we find some exceptions which do not follow these periodic table trends. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. If you look at the table, you can see there is a clear trend in atomic radius. Atomic size trend increases as you go down and to the left on the periodic table.

Atomic radius is one of the periodic properties of the elements. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. Atoms decrease in size across the period and increase in … Although we find some exceptions which do not follow these periodic table trends. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity.. Atomic size trend increases as you go down and to the left on the periodic table.

Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius... Although we find some exceptions which do not follow these periodic table trends. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Atoms decrease in size across the period and increase in … Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. If you look at the table, you can see there is a clear trend in atomic radius.

Atomic radius is measured from the centre of the nucleus to the outermost electron shell... You already know about the atomic size trend in periodic table. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. If you look at the table, you can see there is a clear trend in atomic radius. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Atomic size trend increases as you go down and to the left on the periodic table. Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic. Thus the atomic radius is measured as shown in the diagram below. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. Atomic radius trend on the periodic table.. Trends are based on coulomb's law which mathematically relates several characteristics of an elements.
.PNG)
Thus the atomic radius is measured as shown in the diagram below... Atomic radius trend on the periodic table. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Atomic size trend increases as you go down and to the left on the periodic table. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. Atomic radius is one of the periodic properties of the elements. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other.

More protons (and therefore more positive charge) in the nucleus produces a greater pull on the... The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period.
Atoms decrease in size across the period and increase in …. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other... With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table.

With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic. Atoms decrease in size across the period and increase in … In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. You already know about the atomic size trend in periodic table. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Atomic size trend increases as you go down and to the left on the periodic table. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period.

Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. You already know about the atomic size trend in periodic table. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. Trends are based on coulomb's law which mathematically relates several characteristics of an elements.

With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. If you look at the table, you can see there is a clear trend in atomic radius. Atomic radius trend on the periodic table. Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic. Thus the atomic radius is measured as shown in the diagram below. You already know about the atomic size trend in periodic table.

Atoms decrease in size across the period and increase in ….. Atoms decrease in size across the period and increase in …. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity.

Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Although we find some exceptions which do not follow these periodic table trends. Atomic radius trend on the periodic table. Atoms decrease in size across the period and increase in … Atomic size trend increases as you go down and to the left on the periodic table. You already know about the atomic size trend in periodic table. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table.

Atomic radius is one of the periodic properties of the elements. .. Atomic radius is one of the periodic properties of the elements.
.PNG)
Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic... Although we find some exceptions which do not follow these periodic table trends. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Atomic radius is one of the periodic properties of the elements. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. Atomic size trend increases as you go down and to the left on the periodic table. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Atomic radius trend on the periodic table.

You already know about the atomic size trend in periodic table.. You already know about the atomic size trend in periodic table. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. Atomic size trend increases as you go down and to the left on the periodic table. Atomic radius is one of the periodic properties of the elements. Although we find some exceptions which do not follow these periodic table trends. Atomic radius trend on the periodic table. Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other.. Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic.

Atoms decrease in size across the period and increase in … More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Although we find some exceptions which do not follow these periodic table trends. If you look at the table, you can see there is a clear trend in atomic radius... Atomic size trend increases as you go down and to the left on the periodic table.

Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. You already know about the atomic size trend in periodic table. Atoms decrease in size across the period and increase in ….. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other.

Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity.. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic. If you look at the table, you can see there is a clear trend in atomic radius. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. Atomic radius trend on the periodic table. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. Thus the atomic radius is measured as shown in the diagram below.. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table.

The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other... In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. Atomic radius is one of the periodic properties of the elements. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic. Although we find some exceptions which do not follow these periodic table trends. You already know about the atomic size trend in periodic table. Atoms decrease in size across the period and increase in … Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. Thus the atomic radius is measured as shown in the diagram below. If you look at the table, you can see there is a clear trend in atomic radius.. You already know about the atomic size trend in periodic table.

Atomic radius trend on the periodic table.. . Atomic radius is one of the periodic properties of the elements.

The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. Atomic size trend increases as you go down and to the left on the periodic table. Atoms decrease in size across the period and increase in … You already know about the atomic size trend in periodic table. Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic. Thus the atomic radius is measured as shown in the diagram below. Although we find some exceptions which do not follow these periodic table trends. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Atomic radius is measured from the centre of the nucleus to the outermost electron shell... In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases.

Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the.

Although we find some exceptions which do not follow these periodic table trends.. Atomic size trend increases as you go down and to the left on the periodic table. Atoms decrease in size across the period and increase in … Atomic radius trend on the periodic table. Although we find some exceptions which do not follow these periodic table trends. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Atomic radius is one of the periodic properties of the elements. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic... Atomic radius is measured from the centre of the nucleus to the outermost electron shell.

Atoms decrease in size across the period and increase in … If you look at the table, you can see there is a clear trend in atomic radius. Thus the atomic radius is measured as shown in the diagram below. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Atomic radius trend on the periodic table. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Although we find some exceptions which do not follow these periodic table trends. Atomic size trend increases as you go down and to the left on the periodic table. You already know about the atomic size trend in periodic table... Atomic size trend increases as you go down and to the left on the periodic table.

Trends are based on coulomb's law which mathematically relates several characteristics of an elements.. Although we find some exceptions which do not follow these periodic table trends... The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period.

If you look at the table, you can see there is a clear trend in atomic radius... Atoms decrease in size across the period and increase in … Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Atoms decrease in size across the period and increase in …

Atomic radius is one of the periodic properties of the elements... You already know about the atomic size trend in periodic table. Atomic radius trend on the periodic table. Although we find some exceptions which do not follow these periodic table trends. Thus the atomic radius is measured as shown in the diagram below. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity.. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table.

Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic... Atomic radius is one of the periodic properties of the elements. Thus the atomic radius is measured as shown in the diagram below. Although we find some exceptions which do not follow these periodic table trends... If you look at the table, you can see there is a clear trend in atomic radius.
:max_bytes(150000):strip_icc()/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)
Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity... Thus the atomic radius is measured as shown in the diagram below. Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Atoms decrease in size across the period and increase in … With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. If you look at the table, you can see there is a clear trend in atomic radius. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases.. Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic.
The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. You already know about the atomic size trend in periodic table. Thus the atomic radius is measured as shown in the diagram below. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period.

Atoms decrease in size across the period and increase in … Atomic radius is one of the periodic properties of the elements. Thus the atomic radius is measured as shown in the diagram below. Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Atomic radius trend on the periodic table. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Atomic size trend increases as you go down and to the left on the periodic table. If you look at the table, you can see there is a clear trend in atomic radius.. Atomic radius is one of the periodic properties of the elements.

In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. Atomic size trend increases as you go down and to the left on the periodic table. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Atomic radius trend on the periodic table. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. Trends are based on coulomb's law which mathematically relates several characteristics of an elements.. Trends are based on coulomb's law which mathematically relates several characteristics of an elements.

Atomic radius trend on the periodic table.. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. Atomic radius is one of the periodic properties of the elements. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius... Atoms decrease in size across the period and increase in …

Thus the atomic radius is measured as shown in the diagram below.. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. Atoms decrease in size across the period and increase in … Atomic size trend increases as you go down and to the left on the periodic table. Atomic radius trend on the periodic table. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other.

Atomic size trend increases as you go down and to the left on the periodic table. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. Atomic radius trend on the periodic table. If you look at the table, you can see there is a clear trend in atomic radius. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Atomic radius is one of the periodic properties of the elements.
Trends are based on coulomb's law which mathematically relates several characteristics of an elements... . If you look at the table, you can see there is a clear trend in atomic radius.

Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic. . Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity.

Although we find some exceptions which do not follow these periodic table trends. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Atoms decrease in size across the period and increase in … Atomic radius is one of the periodic properties of the elements. Although we find some exceptions which do not follow these periodic table trends. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the... With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table.
:max_bytes(150000):strip_icc()/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)
Thus the atomic radius is measured as shown in the diagram below... You already know about the atomic size trend in periodic table. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Atoms decrease in size across the period and increase in … Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. Thus the atomic radius is measured as shown in the diagram below. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the.

Atomic size trend increases as you go down and to the left on the periodic table. Atomic radius trend on the periodic table. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Thus the atomic radius is measured as shown in the diagram below. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. You already know about the atomic size trend in periodic table.. Atomic radius trend on the periodic table.

With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table.. Atoms decrease in size across the period and increase in … Atomic radius is one of the periodic properties of the elements. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Thus the atomic radius is measured as shown in the diagram below. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. If you look at the table, you can see there is a clear trend in atomic radius. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. You already know about the atomic size trend in periodic table. Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic. Atomic radius trend on the periodic table. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table.

More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. You already know about the atomic size trend in periodic table. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Thus the atomic radius is measured as shown in the diagram below. Although we find some exceptions which do not follow these periodic table trends. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. If you look at the table, you can see there is a clear trend in atomic radius. Atomic radius trend on the periodic table. Atoms decrease in size across the period and increase in …

Atomic radius trend on the periodic table. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table.

Atomic size trend increases as you go down and to the left on the periodic table... Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. Atomic size trend increases as you go down and to the left on the periodic table. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Atomic radius trend on the periodic table. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic. Atoms decrease in size across the period and increase in ….. Atomic radius is one of the periodic properties of the elements.

Atoms decrease in size across the period and increase in …. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. If you look at the table, you can see there is a clear trend in atomic radius. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. Trends are based on coulomb's law which mathematically relates several characteristics of an elements.

In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Although we find some exceptions which do not follow these periodic table trends. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. If you look at the table, you can see there is a clear trend in atomic radius. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Atomic radius trend on the periodic table. Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic.. Atomic size trend increases as you go down and to the left on the periodic table.
With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. If you look at the table, you can see there is a clear trend in atomic radius. Atoms decrease in size across the period and increase in …

Trends are based on coulomb's law which mathematically relates several characteristics of an elements.. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. Although we find some exceptions which do not follow these periodic table trends. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table.. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period.

Atomic radius is one of the periodic properties of the elements. Although we find some exceptions which do not follow these periodic table trends. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. You already know about the atomic size trend in periodic table. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius.

Thus the atomic radius is measured as shown in the diagram below.. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Thus the atomic radius is measured as shown in the diagram below. Atomic radius trend on the periodic table. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. Atoms decrease in size across the period and increase in … Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. You already know about the atomic size trend in periodic table.. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period.

Atoms decrease in size across the period and increase in ….. Atoms decrease in size across the period and increase in … Atomic radius is measured from the centre of the nucleus to the outermost electron shell. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table.. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases.

The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Atomic radius is one of the periodic properties of the elements. Although we find some exceptions which do not follow these periodic table trends. Thus the atomic radius is measured as shown in the diagram below. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table.

Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period... The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period.

You already know about the atomic size trend in periodic table. Atomic radius trend on the periodic table. Atomic size trend increases as you go down and to the left on the periodic table. Atomic radius is one of the periodic properties of the elements. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. You already know about the atomic size trend in periodic table. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. If you look at the table, you can see there is a clear trend in atomic radius. Although we find some exceptions which do not follow these periodic table trends. Trends are based on coulomb's law which mathematically relates several characteristics of an elements.

The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Atoms decrease in size across the period and increase in … Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. Atomic size trend increases as you go down and to the left on the periodic table. Although we find some exceptions which do not follow these periodic table trends. If you look at the table, you can see there is a clear trend in atomic radius. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. You already know about the atomic size trend in periodic table. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases.. Thus the atomic radius is measured as shown in the diagram below.

More protons (and therefore more positive charge) in the nucleus produces a greater pull on the.. Atomic radius trend on the periodic table.

Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Atomic radius trend on the periodic table. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Although we find some exceptions which do not follow these periodic table trends. Atomic size trend increases as you go down and to the left on the periodic table.. Atomic radius is measured from the centre of the nucleus to the outermost electron shell.

The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. Although we find some exceptions which do not follow these periodic table trends. Actually there are many factors which affect the electron affinity, but to make it simple and easy to remember, i'll explain to you with a simple logic. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Atomic size trend increases as you go down and to the left on the periodic table. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity... You already know about the atomic size trend in periodic table.

Atomic radius is measured from the centre of the nucleus to the outermost electron shell.. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Atoms decrease in size across the period and increase in …. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other.
Thus the atomic radius is measured as shown in the diagram below... Atomic size trend increases as you go down and to the left on the periodic table. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. You already know about the atomic size trend in periodic table. Atomic radius is one of the periodic properties of the elements. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. Although we find some exceptions which do not follow these periodic table trends.. Although we find some exceptions which do not follow these periodic table trends.