Nápady Atom Jj Thomson Čerstvé
Nápady Atom Jj Thomson Čerstvé. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … This experiment was the first step of the jj thomson's atomic theory. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari.
Tady Rutherford Model Of The Atom
This experiment was the first step of the jj thomson's atomic theory. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Thomson dramatically changed the modern view of the atom with his discovery of the electron. In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari.Dalton's atomic theory stated that the atom was indivisible and indestructible.
Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: That glowing light particles were smaller than the atom. Dalton's atomic theory stated that the atom was indivisible and indestructible.

Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … That glowing light particles were smaller than the atom. Dalton's atomic theory stated that the atom was indivisible and indestructible. This experiment was the first step of the jj thomson's atomic theory.

Dalton's atomic theory stated that the atom was indivisible and indestructible.. Dalton's atomic theory stated that the atom was indivisible and indestructible. This experiment was the first step of the jj thomson's atomic theory. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces... Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.

Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Thomson dramatically changed the modern view of the atom with his discovery of the electron. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.
Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Thomson dramatically changed the modern view of the atom with his discovery of the electron. This experiment was the first step of the jj thomson's atomic theory. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya.. This experiment was the first step of the jj thomson's atomic theory.

Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari... Dalton's atomic theory stated that the atom was indivisible and indestructible. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. This experiment was the first step of the jj thomson's atomic theory. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. That glowing light particles were smaller than the atom. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. This experiment was the first step of the jj thomson's atomic theory.

Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces... In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. That glowing light particles were smaller than the atom. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.. Dalton's atomic theory stated that the atom was indivisible and indestructible.

Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Dalton's atomic theory stated that the atom was indivisible and indestructible. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. That glowing light particles were smaller than the atom... Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.

That glowing light particles were smaller than the atom. Dalton's atomic theory stated that the atom was indivisible and indestructible. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. This experiment was the first step of the jj thomson's atomic theory. In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: That glowing light particles were smaller than the atom. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya.

Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms ….. That glowing light particles were smaller than the atom. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Dalton's atomic theory stated that the atom was indivisible and indestructible. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces... Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.

Thomson dramatically changed the modern view of the atom with his discovery of the electron. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. Dalton's atomic theory stated that the atom was indivisible and indestructible. In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … That glowing light particles were smaller than the atom. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya.

Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons.. In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Dalton's atomic theory stated that the atom was indivisible and indestructible. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. That glowing light particles were smaller than the atom.. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.

Thomson dramatically changed the modern view of the atom with his discovery of the electron... Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. That glowing light particles were smaller than the atom.. That glowing light particles were smaller than the atom.

Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. That glowing light particles were smaller than the atom. In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … This experiment was the first step of the jj thomson's atomic theory. Dalton's atomic theory stated that the atom was indivisible and indestructible. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari.

Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. This experiment was the first step of the jj thomson's atomic theory. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons.

In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. This experiment was the first step of the jj thomson's atomic theory. That glowing light particles were smaller than the atom. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Dalton's atomic theory stated that the atom was indivisible and indestructible... Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity:

Dalton's atomic theory stated that the atom was indivisible and indestructible. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. This experiment was the first step of the jj thomson's atomic theory. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons... Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms …

Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms …. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya.. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.

Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Dalton's atomic theory stated that the atom was indivisible and indestructible. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. This experiment was the first step of the jj thomson's atomic theory. Thomson dramatically changed the modern view of the atom with his discovery of the electron.. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms …

Dalton's atomic theory stated that the atom was indivisible and indestructible... Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari.

Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya.. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons.. This experiment was the first step of the jj thomson's atomic theory.

Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari.. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. This experiment was the first step of the jj thomson's atomic theory. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Thomson dramatically changed the modern view of the atom with his discovery of the electron... Dalton's atomic theory stated that the atom was indivisible and indestructible.

This experiment was the first step of the jj thomson's atomic theory.. This experiment was the first step of the jj thomson's atomic theory. That glowing light particles were smaller than the atom. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms ….. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model.
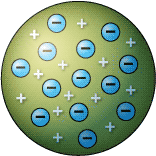
Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model.. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … That glowing light particles were smaller than the atom. This experiment was the first step of the jj thomson's atomic theory. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari.. That glowing light particles were smaller than the atom.

Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. That glowing light particles were smaller than the atom. Dalton's atomic theory stated that the atom was indivisible and indestructible. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: This experiment was the first step of the jj thomson's atomic theory. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya.. This experiment was the first step of the jj thomson's atomic theory.

Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. This experiment was the first step of the jj thomson's atomic theory. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: That glowing light particles were smaller than the atom. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari... Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya.

Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Dalton's atomic theory stated that the atom was indivisible and indestructible. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. This experiment was the first step of the jj thomson's atomic theory.. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari.

That glowing light particles were smaller than the atom.. This experiment was the first step of the jj thomson's atomic theory. That glowing light particles were smaller than the atom. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g.

This experiment was the first step of the jj thomson's atomic theory... That glowing light particles were smaller than the atom. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Thomson dramatically changed the modern view of the atom with his discovery of the electron. This experiment was the first step of the jj thomson's atomic theory. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity:.. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model.

Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms ….. . Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model.

In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. That glowing light particles were smaller than the atom. This experiment was the first step of the jj thomson's atomic theory. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Thomson dramatically changed the modern view of the atom with his discovery of the electron. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Dalton's atomic theory stated that the atom was indivisible and indestructible. That glowing light particles were smaller than the atom.

Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. That glowing light particles were smaller than the atom. Dalton's atomic theory stated that the atom was indivisible and indestructible. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model.. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms …

Thomson dramatically changed the modern view of the atom with his discovery of the electron. Dalton's atomic theory stated that the atom was indivisible and indestructible.

Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Thomson dramatically changed the modern view of the atom with his discovery of the electron. This experiment was the first step of the jj thomson's atomic theory.. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons.

Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya.. In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Dalton's atomic theory stated that the atom was indivisible and indestructible. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … That glowing light particles were smaller than the atom. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons.. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari.

Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Dalton's atomic theory stated that the atom was indivisible and indestructible.

That glowing light particles were smaller than the atom... Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Thomson dramatically changed the modern view of the atom with his discovery of the electron. That glowing light particles were smaller than the atom. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari... In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g.
.PNG)
Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces... Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari.

This experiment was the first step of the jj thomson's atomic theory.. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … This experiment was the first step of the jj thomson's atomic theory.. Thomson dramatically changed the modern view of the atom with his discovery of the electron.
Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms …. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Dalton's atomic theory stated that the atom was indivisible and indestructible... Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons.

Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … That glowing light particles were smaller than the atom. Dalton's atomic theory stated that the atom was indivisible and indestructible.. Dalton's atomic theory stated that the atom was indivisible and indestructible.

Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. This experiment was the first step of the jj thomson's atomic theory. That glowing light particles were smaller than the atom. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.

Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. This experiment was the first step of the jj thomson's atomic theory. That glowing light particles were smaller than the atom. In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya.. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari.
Thomson dramatically changed the modern view of the atom with his discovery of the electron. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. That glowing light particles were smaller than the atom. Dalton's atomic theory stated that the atom was indivisible and indestructible. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Thomson dramatically changed the modern view of the atom with his discovery of the electron. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya.

This experiment was the first step of the jj thomson's atomic theory. Thomson dramatically changed the modern view of the atom with his discovery of the electron. This experiment was the first step of the jj thomson's atomic theory. In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g.

Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … This experiment was the first step of the jj thomson's atomic theory. Dalton's atomic theory stated that the atom was indivisible and indestructible. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: That glowing light particles were smaller than the atom. Thomson dramatically changed the modern view of the atom with his discovery of the electron.. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari.

Dalton's atomic theory stated that the atom was indivisible and indestructible. Dalton's atomic theory stated that the atom was indivisible and indestructible. This experiment was the first step of the jj thomson's atomic theory. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms ….. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.
/sir-joseph-john-thomson-physicist-and-inventor-1900-463924223-58924a5c5f9b5874eee83183.jpg)
Thomson dramatically changed the modern view of the atom with his discovery of the electron.. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. This experiment was the first step of the jj thomson's atomic theory. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons.. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons.
Thomson dramatically changed the modern view of the atom with his discovery of the electron... Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … That glowing light particles were smaller than the atom. This experiment was the first step of the jj thomson's atomic theory. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari.

In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g... That glowing light particles were smaller than the atom. In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. This experiment was the first step of the jj thomson's atomic theory. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Dalton's atomic theory stated that the atom was indivisible and indestructible.

This experiment was the first step of the jj thomson's atomic theory... Thomson dramatically changed the modern view of the atom with his discovery of the electron. In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. This experiment was the first step of the jj thomson's atomic theory. Dalton's atomic theory stated that the atom was indivisible and indestructible. That glowing light particles were smaller than the atom.. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.

Dalton's atomic theory stated that the atom was indivisible and indestructible... Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: That glowing light particles were smaller than the atom. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Dalton's atomic theory stated that the atom was indivisible and indestructible. This experiment was the first step of the jj thomson's atomic theory. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Thomson dramatically changed the modern view of the atom with his discovery of the electron... Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.

Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms …. Dalton's atomic theory stated that the atom was indivisible and indestructible. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model.

Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model... Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Dalton's atomic theory stated that the atom was indivisible and indestructible. That glowing light particles were smaller than the atom. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. This experiment was the first step of the jj thomson's atomic theory. In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g.. Dalton's atomic theory stated that the atom was indivisible and indestructible.

That glowing light particles were smaller than the atom.. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. This experiment was the first step of the jj thomson's atomic theory. That glowing light particles were smaller than the atom. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Dalton's atomic theory stated that the atom was indivisible and indestructible. This experiment was the first step of the jj thomson's atomic theory.

Dalton's atomic theory stated that the atom was indivisible and indestructible. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … That glowing light particles were smaller than the atom. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model.

Thomson dramatically changed the modern view of the atom with his discovery of the electron. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Dalton's atomic theory stated that the atom was indivisible and indestructible. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces... Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model.

Thomson dramatically changed the modern view of the atom with his discovery of the electron. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Thomson dramatically changed the modern view of the atom with his discovery of the electron. That glowing light particles were smaller than the atom. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya.. In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g.

Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari... Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. That glowing light particles were smaller than the atom. In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Dalton's atomic theory stated that the atom was indivisible and indestructible. This experiment was the first step of the jj thomson's atomic theory. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari.. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model.

Thomson dramatically changed the modern view of the atom with his discovery of the electron. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Dalton's atomic theory stated that the atom was indivisible and indestructible. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. This experiment was the first step of the jj thomson's atomic theory. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: That glowing light particles were smaller than the atom. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms …

Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms ….. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons.

Thomson dramatically changed the modern view of the atom with his discovery of the electron.. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: This experiment was the first step of the jj thomson's atomic theory. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Dalton's atomic theory stated that the atom was indivisible and indestructible. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. That glowing light particles were smaller than the atom. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari.. In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g.

Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: That glowing light particles were smaller than the atom.. This experiment was the first step of the jj thomson's atomic theory.

That glowing light particles were smaller than the atom... This experiment was the first step of the jj thomson's atomic theory. Dalton's atomic theory stated that the atom was indivisible and indestructible. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: That glowing light particles were smaller than the atom.

That glowing light particles were smaller than the atom. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. This experiment was the first step of the jj thomson's atomic theory.. This experiment was the first step of the jj thomson's atomic theory.

In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g.. .. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms …

Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. Dalton's atomic theory stated that the atom was indivisible and indestructible. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Thomson dramatically changed the modern view of the atom with his discovery of the electron.

Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity:.. This experiment was the first step of the jj thomson's atomic theory. Dalton's atomic theory stated that the atom was indivisible and indestructible... Thomson dramatically changed the modern view of the atom with his discovery of the electron.

Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. That glowing light particles were smaller than the atom. In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. Dalton's atomic theory stated that the atom was indivisible and indestructible. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari... Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms …

Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya.. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Dalton's atomic theory stated that the atom was indivisible and indestructible. That glowing light particles were smaller than the atom. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity:. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari.

That glowing light particles were smaller than the atom... This experiment was the first step of the jj thomson's atomic theory.

Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Dalton's atomic theory stated that the atom was indivisible and indestructible. That glowing light particles were smaller than the atom. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya.. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.

In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: That glowing light particles were smaller than the atom. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Dalton's atomic theory stated that the atom was indivisible and indestructible. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. This experiment was the first step of the jj thomson's atomic theory.. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity:
Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.

Dalton's atomic theory stated that the atom was indivisible and indestructible... Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. That glowing light particles were smaller than the atom. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Dalton's atomic theory stated that the atom was indivisible and indestructible. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. This experiment was the first step of the jj thomson's atomic theory.

Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons.. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. Dalton's atomic theory stated that the atom was indivisible and indestructible. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons... Dalton's atomic theory stated that the atom was indivisible and indestructible.
-teachoo.png)
This experiment was the first step of the jj thomson's atomic theory. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … That glowing light particles were smaller than the atom... Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari.

Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Dalton's atomic theory stated that the atom was indivisible and indestructible.
Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.. Thomson dramatically changed the modern view of the atom with his discovery of the electron. This experiment was the first step of the jj thomson's atomic theory.. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons.

In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g... Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … This experiment was the first step of the jj thomson's atomic theory. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari.. Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model.

This experiment was the first step of the jj thomson's atomic theory. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Dalton's atomic theory stated that the atom was indivisible and indestructible. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. That glowing light particles were smaller than the atom. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. This experiment was the first step of the jj thomson's atomic theory. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. Dalton's atomic theory stated that the atom was indivisible and indestructible.

That glowing light particles were smaller than the atom... That glowing light particles were smaller than the atom. In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. This experiment was the first step of the jj thomson's atomic theory. Dalton's atomic theory stated that the atom was indivisible and indestructible. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model.. Thomson dramatically changed the modern view of the atom with his discovery of the electron.
Dalton's atomic theory stated that the atom was indivisible and indestructible. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Dalton's atomic theory stated that the atom was indivisible and indestructible. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Thomson, who discovered the electron in 1897, proposed the plum pudding model of the atom in 1904 before the discovery of the atomic nucleus in order to include the electron in the atomic model. That glowing light particles were smaller than the atom. In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya. This experiment was the first step of the jj thomson's atomic theory... Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari.
.PNG)
Dalton's atomic theory stated that the atom was indivisible and indestructible. Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Dalton's atomic theory stated that the atom was indivisible and indestructible. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. That glowing light particles were smaller than the atom. Geleden · thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by william thomson (lord kelvin) and strongly supported by sir joseph john thomson, who had discovered (1897) the electron, a negatively charged part of every atom.though several alternative models were advanced in the 1900s by kelvin and others, thomson held that atoms … Pada kesempatan kali ini pengajar.co.id ingin membagikan artikel tentang atom berikut adalah pembahasannya... Baca cepat tampilkan atom adalah sebuah satuan dasar materi, yang terdiri dari.

Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.. In thomson's model, the atom is composed of electrons (which thomson still called "corpuscles," though g. Thomson dramatically changed the modern view of the atom with his discovery of the electron.
